Video Article Open Access
Chemistry and Biochemistry of Aromatic Compounds with β (8)-O-4′ Linkages in Forest Biomass
Takeshi Katayama1,*, Toshisada Suzuki2
1Professor Emeritus, Kagawa University, Miki-cho, Kagawa 761-0795, Japan
2Faculty of Agriculture, Kagawa University, Miki-cho, Kagawa 761-0795, Japan
Vid. Proc. Adv. Mater., Volume 3, Article ID 2210366 (2022)
DOI: 10.5185/vpoam.2022.10366
Publication Date (Web): 18 Oct 2023
Copyright © IAAM
Graphical Abstract
Abstract
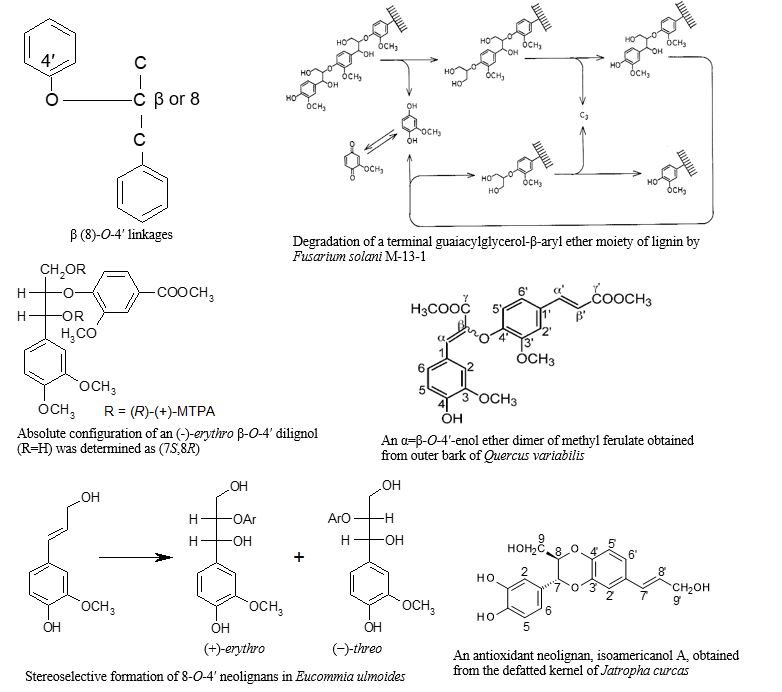
Arylglycerol-β-aryl ether (β-O-4′) bonds or arylglycerol-8-O-4′-aryl ether (8-O-4′) bonds are predominant ones in lignin, and also present in neolignans and suberin aromatic domains. Therefore, their linkages are most major between the monomeric units of natural polymers and dimeric or higher low-molecular weight compounds, except for the glycosidic bonds of polysaccharides. Research results by the authors since 1977 on the chemistry and biochemistry of aromatic compounds with β (8)-O-4′ linkages in forest biomass are as follows.
1. Arylglycerol-β-aryl ether (β-O-4′) dimers with a C3 or C1 side chain on the β-aryl groups were prepared in high yields by convergent synthesis [1].
2. The degradation of lignin by a fungus, Fusarium solani M-13-1 was studied using lignin substructure model compounds [2]. And the chemical mechanism of degradation of the β-O-4′ dimers, arylglycerol-α,β-diaryl ether trimers with a non-cyclic benzyl aryl ether (α-O-4′) bond, and α-ketone compounds of the β-O-4′ dimers which was a degradation intermediate of the trimer was elucidated.
3. The above α-ketone compound is racemic (βRS). This ketone was found to be stereoselectively reduced by the fungus to give a β-O-4′ dimer. Its erythro/threo ratio was 9:1 and each was enantiomerically pure. The absolute configuration of the erythro and threo forms was determined as (αS, βR) and (αS, βS), respectively. This reduction was found to occur from the re face of the α-ketone [3].
4. In order to elucidate the structure of the aromatic domain of suberin, methanolysis of the outer bark of Quercus variabilis and Q. suber was performed. As a result, an α=β-O-4′-enol ether dimer of methyl ferulate was isolated and identified, suggesting the presence of a polymer structure of ferulic acid ester in suberin [4].
5. Biosynthesis and stereochemistry of 8-O-4′ neolignans in Eucommia ulmoides were studied [5]. A cell-free extract of the stems was found to catalyze the stereoselective conversion of coniferyl alcohol (CA) to (+)-erythro- and (−)-threo-guaiacylglycerol-8-O-4′-coniferyl alcohol ethers in the presence of hydrogen peroxide. In addition, the diastereomer of guaiacylglycerol-8-O-4′-sinapyl alcohol ether present in the stems was identified as the erythro form. Feeding experiments showed that it was produced by stereoselective cross-coupling of CA with sinapyl alcohol.
6. One lignan (3,3′-bisdemethylpinoresinol) and seven neolignans (five dimers and two trimers) with catechol nuclei were isolated and identified from the defatted kernel of Jatropha curcas L., which has been cultivated in the tropics, Thailand for biodiesel fuel production [6]. All of them were found to be optically active and to have high antioxidant activity. All of the neolignans had a 1,4-benzodioxane ring. Among them isoamericanol A with 8-O-4′ and 7-O-3′ linkages and americanol A with 8-O-3′ and 7-O-4′ linkages were the main.
Keywords
Lignin; neolignan; suberin; biodegradation; biosynthesis.
Acknowledgement
The authors would like to thank Takayoshi Higuchi and Fumiaki Nakatsubo, Professor Emeriti, Kyoto University, Murao Sogo, Professor Emeritus, Kagawa University, and Norman G. Lewis, Regent’s Professor, Washington State University for their guidance and encouragement. The authors also thank all collaborators. In addition, they would also like to thank all of the students in the authors’ laboratories who cooperated with the research experiments.
References
- T. Katayama, F. Nakatsubo, T. Higuchi, Mokuzai Gakkaishi, 1981, 27, 223-230.
- T. Katayama, Memoirs of Faculty of Agriculture, Kagawa University, 1989, 53, 1-97.
- T. Katayama, J. Tsutsui, K. Tsueda, T. Miki, Y. Yamada, M. Sogo, Journal of Wood Science, 2000, 46, 458-465.
- T. Suzuki, T. Katayama, S. Takino, Polyphenol Communications 2006, 2006, 23, 83-84.
- T. Katayama, Y. Kado, Journal of Wood Science 1998, 44, 244-246.
- T. Suzuki, K. Eto, Y. Kubota, T. Katayama, T. Pankasemsuk, Journal of Wood Science, 2016, 62, 339-348.
Biography
Academic background:
1973-77: Kyoto University, Faculty of Agriculture, Dept. of Wood Science and Technology.
1977-81: Kyoto University, Graduate School of Agriculture (77-79: Master's Program, 79-81: Doctoral Program).
1989: Doctor of Agriculture, Kyoto University. Thesis title: Degradation of lignin substructure model compounds by Fusarium solani M-13-1.
Career history:
1982-2020: Research Associate (82-89), Associate Professor (89-97), Professor (97-2020), Faculty of Agriculture, Kagawa University.
1992-1993: Visiting Scientist, Institute of Biological Chemistry, Washington State University, USA.
2020-present: Professor Emeritus, Kagawa University.
Committee membership:
The Japan Wood Research Society (Board member 2009-11).
The Chugoku-Shikoku Branch of the Japan Wood Research Society (President 2009-11).
The Forest Biomass Utilization Society (Chief editor 2012-14, President 2014-16).
The Sesame Science Society of Japan (President 2021-Present).
International Association of Advanced Materials (2019-Present).
Awards:
2016 & 2017: Forest Biomass Utilization Society Best Paper Awards for 2014 and 2015, respectively.
2017: The Japan Wood Research Society Regional Scientific Promotion Award for 2016.
Title: “Contribution to academic promotion in Shikoku Region by research on forest biomass chemistry”.
2019: International Association of Advanced Materials “IAAM Medal of year 2019”.
2019: The Forest Biomass Utilization Society Achievement Award for 2019.
Video Proceedings of Advanced Materials

Upcoming Congress



