Video Article Open Access
Development of New Cyclometalated Iridium(III) Complexes for Induction of Programmed Cell Death, Detection of Dead Cells, and Mechanistic Study
Shin Aoki1,2,3,* Kenta Yokoi1, Chandrasekar Balachandran1, Kohei Yamaguchi1
1Faculty of Pharmaceutical Sciences, Tokyo University of Science, Noda, 278-8510, Japan
2Research Institute for Science and technology, Tokyo University of Science, Noda, 278-8510, Japan
3Research Institute for Biomedical Sciences, Tokyo University of Science, Noda, 278-8510, Japan
Vid. Proc. Adv. Mater., Volume 3, Article ID 2208312 (2022)
DOI: 10.5185/vpoam.2022.08312
Publication Date (Web): 22 Dec 2022
Copyright © IAAM
Graphical Abstract
Abstract
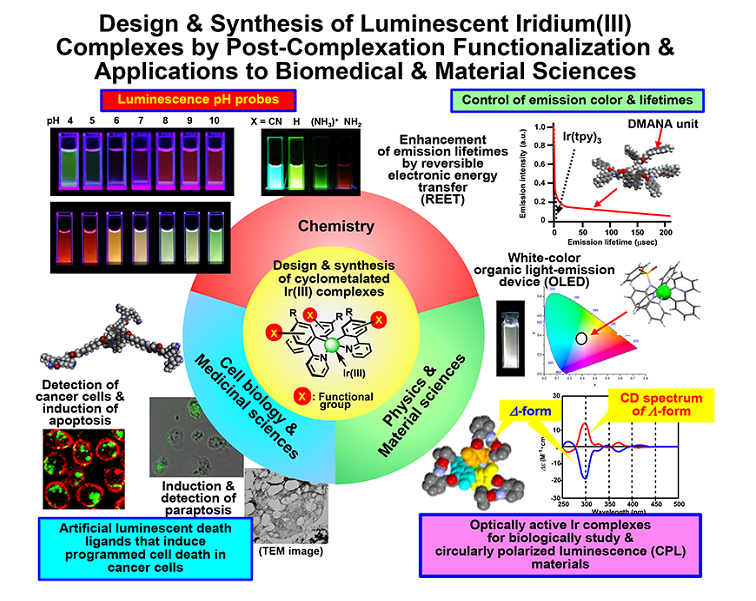
Cyclometalated iridium(III) (Ir(III) complexes such as fac-Ir(tolpy)3 (tolpy = 2-(4’-tolyl)pyridine) are extremely stable in water and organic solvents and are used as organic light emission diodes (OLEDs) and cell imaging materials, due to their high stability and excellent emission properties such as large Stokes’ shifts, high phosphorescent quantum yields, and relatively longer lifetimes (ms order) than that of many fluorescent molecules (ns order). These attractive photochemical and structural properties make cyclometalated Ir complexes important candidates for the use in biological applications such as chemosensors, photocatalysts in organic synthetic chemistry, living cell staining, in vivo tumor imaging, and anti-cancer agents. Because it is generally difficult to synthesize various metal complexes that contain labile and/or unstable functional groups under harsh reaction conditions (such as high temperature), we have developed the synthetic methods for various hybrid compounds of cyclometalated iridium(III) complexes by “post-complexation functionalization (PCF)” including regioselective substitution reactions of basic Ir complexes [1]. It is assumed that these functionalization reactions would be powerful methods for the synthesis of a variety of Ir complexes possessing labile functional groups such as peptides, chromophores as well as other moieties that would otherwise be difficult to produce for the applications to biomedical and material science. In this paper, we would like to present the results of the following three projects.
1) Design and synthesis of cyclometalated Ir(III) complexes that exhibit blue, green, and red color luminescence emission and respond to pH in solutions [1].
2) Design and synthesis of luminescent Ir(III) complex-peptide hybrids (IPH) that detects cancer cells and induce their programmed cell death, apoptotis and necrosis, and paraptosis, in cancer cells [2].
3) Design and synthesis of Ir(III) complex-peptide hybrids (IPH) function as inducers of paraptosis in cancer cells and detectors of dead cancer cells [3-5].
Mechanistic studies of cell death induced ty IPHs having peptide units that had been known to bind to death receptors expressed on cell membrane of cancer cells suggest that apoptosis and necrosis-like cell death are differentiated by the position of the hydrophilic linkers between Ir complex core and the peptide units. On the other hand, IPHs that have basic (cationic) peptide units have been found to induce paraptosis, which has recently been reported as one of PCD mechanisms, not apoptosis nor necrosis. Interestingly, it was found that our IPHs induce the direct Ca2+ transfer from the endoplasmic reticulum (ER) to mitochondria, the loss of mitochondria membrane potential, membrane fusion between the ER and mitochondria, and the vacuolization of intracellular organelles in cancer cells [3-5]. Paraptosis induced by IPHs has been proposed as paraptosis-II, which is different from previously known paraptosis (paraptosis-I) induced by celastrol and other natural products, in which the Ca2+ overload in mitochondria is negligibly induced [5]. These findings suggest that IPHs could be a promising tool for controlling apoptosis and necrosis by activation of the extra-and intracellular cell death pathways and to develop new anticancer drugs that detect cancer cells and induce their cell death.
Keywords
Cyclometalated iridium complexes; post-complexation functionalization; hybrid molecules; luminescence property; anticancer activity.
Acknowledgement
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, the Uehara Memorial Foundation, Tokyo University of Science. Research grant from Tokyo Ohka Foundation for the Promotion of Science and Technology, Kanagawa, Japan, and research grant from Tokyo Biochemical Research Foundation, Tokyo, Japan, the scholarship of International Association for the Exchange of Students for Technical Experience (IAESTE), and the TUS (Tokyo University of Science) fund for strategic research area.
References
- S. Aoki, Y. Matsuo, S. Ogura, H. et al., Inorg. Chem., 2011, 50, 806. .
- A.-A. Masum, K. Yokoi, Y. Hisamatsu, K. Naito, B. Shashni, S. Aoki, Bioorg. Med. Chem., 2018, 26, 4804.
- K. Yokoi, C. Balachandran, M. Umezawa, K. Tsuchiya, A. Mitrić, S. Aoki, ACS Omega, 2020, 5, 6983.
- C. Balachandran, K. Yokoi, K. Naito, et al. Molecules 2021, 26, 7028 (32 pages)
- K. Yokoi, K. Yamaguchi, M. Umezawa, K. Tsuchiya, S. Aoki, Biochemistry, 2022, accepted.
Video Proceedings of Advanced Materials

Upcoming Congress



