Video Article Open Access
Water Chemistry and Oxidation State of Mo Affects Dissolution and Visible-light Photocatalytic Activity of MoO3 Nanostructures
Debora F. Rodrigues1,2,*, Janire Peña-Bahamonde1, Sofia K. Fanourakis2
1Department of Civil and Environmental Engineering, University of Houston, Houston, TX 77204 - 4003
2 Department of Materials Science and Engineering, University of Houston, Houston, TX 77204 - 4003
Vid. Proc. Adv. Mater., Volume 2, Article ID 2021-0299 (2021)
DOI: 10.5185/vpoam.2021.0299
Publication Date (Web): 19 Feb 2021
Copyright © IAAM
Graphical Abstract
Abstract
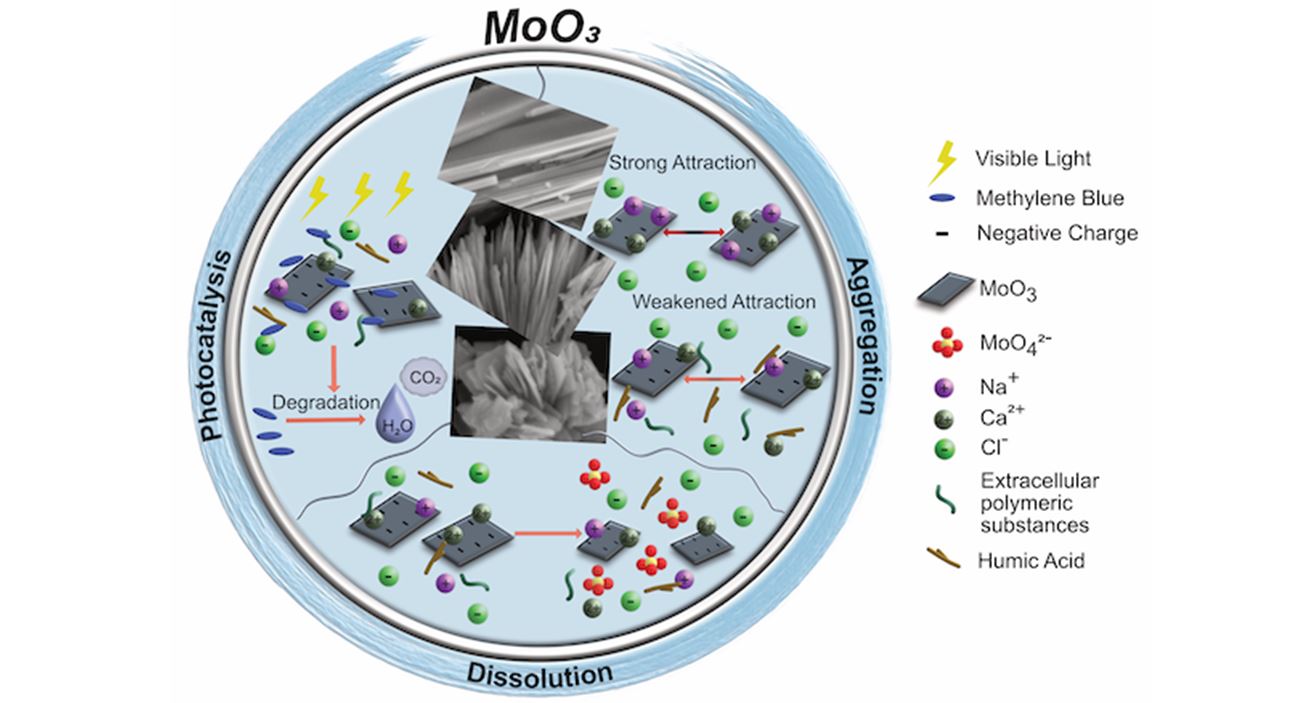
The role of shape, aggregation state, dissolution, and chemical properties of MoO3 nanomaterials with visible light photocatalytic activity is still largely unknown. In the present study, we investigate the effects of inorganic salts (NaCl and CaCl2) and natural organic matter (NOM), such as humic acid (H.A.) and extracellular polymeric substances (EPS), as well as pH on nanoparticle aggregation, dissolution, and ultimately on the photocatalytic properties of three forms of molybdenum trioxide (MoO3), i.e., nanorods, nanowires, and nanoplates. In the presence of NaCl, nanorod, nanowire, and nanoplate MoO3 had similar critical coagulation concentrations, while the nanorods showed higher instability in CaCl2. Overall, inorganic salts’ presence caused high colloidal instability in the MoO3 nanostructures in terms of aggregation behavior but greatly aided in reducing MoO3 dissolution. NOM presence decreased aggregation rates, albeit dissolution was not similarly affected in all three structures. Only the dissolution of the nanowire structures was decreased in the presence of H.A. or EPS. Furthermore, the nanowires and nanoplates’ photocatalytic activities were reduced when inorganic salts or natural organic matter were present. While the presence of natural organic matter alone did reduce the photocatalytic effectiveness of the nanorod MoO3, the presence of salts seemed to negate the effects of the organic compounds. Furthermore, the presence of CaCl2 resulted in a highly enhanced photocatalytic activity regardless of the presence of natural organic matter. The photodegradation properties and role of dissolution products under different pH values were also investigated. We show that different morphologies of MoO3 present different solubility behaviors with increasing pH (with the highest solubility occurring at pH 10), and this dissolution depends on the oxidative state and nature of the Mo-O bonds, not just the size and morphology of the nanostructures. Nanoparticle dissolution seems to favorably affect the discoloration rate of methylene blue (M.B.) but not its photocatalytic degradation. It is essential to differentiate M.B. discoloration as opposed to photocatalytic degradation since discoloration involves not only photocatalytic degradation but also adsorption and ion complexation processes. Our experiments for the removal of M.B. show that the nanorods present the best photocatalytic-based degradation activity, while the nanowires, which present the highest dissolution, decolorize M.B. the fastest. MoO3 photocatalytic degradation mechanism was investigated via the quantification of nanoparticle-produced reactive oxygen species (ROS) and measurement of M.B. photocatalytic degradation inhibition due to the presence of ROS scavengers. According to the results, photogenerated holes in the nanomaterial govern the degradative process by allowing the production of hydrogen peroxide. This study demonstrates that MoO3 nanoparticle chemical and physical properties played a significant role in their aggregation, dissolution, and ability to photocatalytically degrade methylene blue in solution. Furthermore, we demonstrated that the nanoparticle dissolution directly influences the photocatalytic properties of MoO3 nanostructures.
Keywords
Molybdenum trioxide, nanorods, nanowires, nanoplates, photocatalytic activity.
Acknowledgement
This work was supported by the NSF BEINM Grant Number: 1705511, and the Robert A. Welch Foundation award number (E-2011-20190330). The findings achieved herein are solely the responsibility of the authors.
References
- S.F. Fanourakis, J. Peña-Bahamonde, P.C. Bandara, D.F. Rodrigues, npj Clean Water, 2020, 3, 1-15.
- J. Peña-Bahamonde, C. Wu, S.F. Fanourakis, S.M. Louie, J. Bao, D.F. Rodrigues, Journal of Catalysis, 2020, 381, 508-519.
Biography
Debora F. Rodrigues is currently the Ezekiel Cullen Professor of Environmental Engineering at the University of Houston in the Civil and Environment engineering. She also holds a joint appointment with the Materials Sciences and Engineering Department at the University of Houston. Dr. Rodrigues received her B.S. and M.S. from the University of Sao Paulo, Brazil, and her Ph.D. from Michigan State University in 2007. She was a postdoctoral associate in the Environmental Engineering Program at Yale University from 2007 to 2010. Since the beginning of her career at U.H., she was the recipient of several National Awards, such as the National Science Foundation (NSF) Career Award in 2012, the Inaugural Emerging Investigator Research Award in 2014 from the Sustainable Nanotechnology Organization for the high impact of her researcher in nanotechnologies. In 2016, she received two prestigious awards, one from the U.S. Dept. of Energy, the C3E Research Award in water-energy nexus, and the Environmental Award in the 28th HENAAC Conference. In 2017, she was nominated by an NAE member to participate in the Prestigious Frontiers of Engineering (FOE) program of the National Academy of Engineering (NAE) in the U.S. She has published more than 80 peer-reviewed manuscripts in tier one journals and received more than 5500 citations. She is associate editor for two prestigious journals, namely, npj Clean Water journal (Nature publishing group) and the Journal of Hazardous Materials (Elsevier). The central topic of her research interest is in the Health-Energy-Water Nexus. Her research is multidisciplinary and involves environmental engineering, environmental microbiology, and materials sciences and engineering approaches. In her research, she has harvested microorganisms’ abilities to naturally produce materials for different engineering applications or used sustainable nanotechnology approaches to remove microorganisms and chemicals from water.
Video Proceedings of Advanced Materials

Upcoming Congress



