Video Article Open Access
Engineering Amphiphilic Bioresorbable Polymers for Constructing Colloidal Vesicles in the Pursuit of Innovative Vaccine Design
Ming-Hsi Huang
National Institute of Infectious Diseases and Vaccinology, National Health Research Institutes, Miaoli 35053, Taiwan
Vid. Proc. Adv. Mater., Volume 2, Article ID 2021-02136 (2021)
DOI: 10.5185/vpoam.2021.02136
Publication Date (Web): 28 Mar 2021
Copyright © IAAM
Graphical Abstract
Abstract
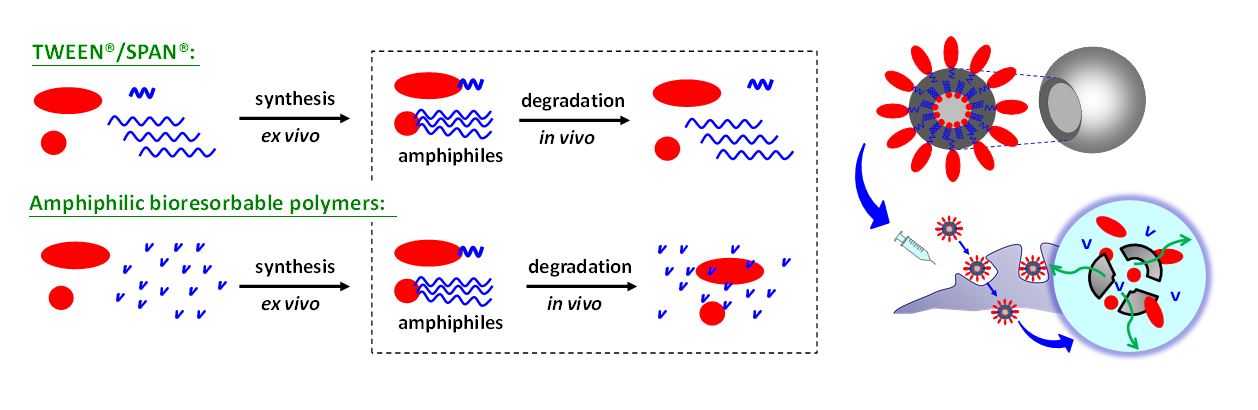
Two key challenges for innovative vaccine design are related to developing formulations that avoid cold chain shipment and finding a delivery vehicle that is absorbable in vivo. Toward this, we systematically engineered amphiphilic bioresorbable polymers for constructing colloidal vesicles and prolonging vaccine delivery in the pursuit of innovative vaccine design. [1] We explored the design and performance of a colloidal vesicle consisting of two immiscible liquids, metabolizable squalene oil and water, which was stabilized by amphiphilic bioresorbable polymers consisting of hydrophilic groups and lipophilic groups. The hydrophilic group was made of sorbitan or polyethylene glycol (PEG); on the other hand, the lipophilic group comprised polyesters derived from lactyl (LA) and caproyl (CL) motifs, which have been widely regarded as degradable polyesters (patents granted for hydrophilic emulsifiers: US 8,444,993, TW I383806; patents granted for lipophilic emulsifiers: TW I598114, US 10,172,945 B2 and AU 2016215757). These amphiphilic bioresorbable polymers act as surface-active agents to stabilize the oil/water interfaces and give rise to oil-shelled polysorbasomes (polymeric absorbable vesicles) during preparation and storage. Among these, we optimized an emulsion-type delivery system comprised of a bioresorbable polymer PEG–PLACL, Span®85, and squalene to form a ready-to-use adjuvant, dubbed PELC. [2-4] The immunogenicity studies assessed in mice using an inactivated influenza viral antigen have demonstrated that PELC allowed for antigen dose sparing and cross-protection, indicating that the use of PELC could be an effective tool for pandemic vaccine preparedness. [2] These advances also offer the potential in the immunotherapeutic treatment for enhancing the cell-mediated responses elicited by human papillomavirus (HPV) antigens to suppress cervical cancer cell growth. [3] Recently, our research group progressively launched a series studies toward the mechanism elucidation of PELC emulsion adjuvant and identified the contribution of each ingredient of PELC linking with vaccine efficacy. First, PELC is degradable; we demonstrated that PELC can reshape the immunogenicity profiles of a model antigen and ameliorate the vaccine efficacy. [4] Our results confirm the loss of PLACL moiety of the emulsifier PEG–PLACL directly affected the stability of PEG–PLACL-stabilized emulsion, leading to emulsion disintegration and squalene/water phase separation; histological examination and biochemistry tests show that formulation with low oil content (5<%) is considerable tolerance via i.m. injection in mice. Second, PELC is double; we aimed to understand how antigen-adjuvant associations influence vaccine efficacy and lead to optimal vaccine formulations. [5] We launched a mouse study highlighted that squalene-cored double emulsion PELC exhibits both immunopotentiator and delivery system. Third, PELC is deformable; such liquid-liquid colloids were soft and deformable so that the size could be controlled by passage through an extruder membrane. We propose that tailoring emulsified particles to monodisperse nanosized distribution plays a critical role in facilitating the transportation of antigen across mucosal membrane to the nasal-associated lymphoid tissue, compared with original polydisperse submicron ones (Patent Application: TW 106137133; US 16/172,207; CN 201711132357.5). In the absence of any immunostimulator, nanoemulsion could rephrase the immunological signatures following intranasal vaccination and induce broad-spectrum antigen-specific cellular immunity. These features provide new insights into the field of sustained delivery based on material engineering.
Keywords
Amphiphilic bioresorbable polymers; emulsions; nasal spray delivery; polysorbasomes; vaccine adjuvants.
Acknowledgement
This work was supported by grant 109A1-IVPP19-014 from the National Health Research Institutes of Taiwan and grant 106-2314-B-400-016-MY3 from the Ministry of Science and Technology (MOST) of Taiwan.
References
- C. Y. Huang, C. H. Huang, S. J. Liu, H. W. Chen, C. H. Leng, P. Chong, M. H. Huang, ACS Applied Materials & Interfaces, 2018, 10, 12553.
- C. H. Huang, C. Y. Huang, C. P. Cheng, S. H. Dai, H. W. Chen, C. H. Leng, P. Chong, S. J. Liu, M. H. Huang, Scientific Reports, 2016, 6, 36732.
- M. H. Huang, S. C. Lin, C. H. Hsiao, H. J. Chao, H. R. Yang, C. C. Liao, P. W. Chuang, H. P. Wu, C. Y. Huang, C. H. Leng, S. J. Liu, H. W. Chen, A. H. Chou, A. Y. C. Hu, P. Chong, PLoS ONE, 2010, 5, e12279.
- Y. C. Song, H. Y. Cheng, C. H. Leng, S. K. Chiang, C. W. Lin, P. Chong, M. H. Huang, S. J. Liu, Journal of Controlled Release, 2014, 173, 158.
- C. H. Huang, C. Y. Huang, M. H. Huang, Biomedicine & Pharmacotherapy, 2019, 118, 109373.
Biography
Ming-Hsi Huang is an Associate Investigator of the NIIDV of NHRI, Taiwan. His major interest is to develop novel biomaterials, in particular bioresorbable polymers and synthetic peptides for applications as substrate in vaccine adjuvants or as scaffolding in tissue engineering. His mission at NIIDV has been focused on the development of novel delivery vehicles for generating vaccine-induced long-term immunity as well as immunoregulatory agents for manipulating effective/harmful immune responses. His research group was the pioneer who engineers amphiphilic bioresorbable polymers for constructing colloidal vesicles in the pursuit of innovative vaccine design. At the outset, the search group studies on the development of micro-encapsulation technology for a single-dose multivalent vaccine against emerging infectious diseases, in particular influenza-associated illness and hand-foot-mouth disease. Currently, he aimed to launch a mechanistic study on how emulsion adjuvants interacting with immune cells and progressively elucidate the role of manufacturing process as well as each ingredient linking with vaccine immunogenicity. He also extends these aspects to optimize an appropriate vaccination route, such as subcutaneous, intramuscular or mucosal administration. These features are of great interest for further investigations on sustained delivery for cancer immunotherapy. He obtained his PhD degree in 2004 in Materials Chemistry from University Montpellier I in France. From 2005-2009, he worked as a postdoctoral fellow in Vaccine Research and Development Center of NHRI. From 2009-2014, he worked as an Assistant Investigator in NIIDV of NHRI. He has published 40 SCI journal articles and granted 4 inventions (total 8 patents in multiple countries). The work accomplished by Dr Huang on vaccination/immunotherapy by formulating candidate vaccines with novel emulsion-type adjuvants has been published in the leading journals in the field of biomaterials/nanomedicine.
Video Proceedings of Advanced Materials

Upcoming Congress



