Video Article Open Access
Importance of Transport in Electrochemical Reactivity
Lien-Chun Weng1,2, Justin Bui1,2, Lalit Pant1, Alexis T. Bell1,2, Adam Z. Weber1,*
1Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
2Department of Chemical and Biomolecular Engineering, University of California, Berkeley, CA 94720, USA
Vid. Proc. Adv. Mater., Volume 2, Article ID 2021-0177 (2021)
DOI: 10.5185/vpoam.2021.0177
Publication Date (Web): 13 Feb 2021
Copyright © IAAM
Graphical Abstract
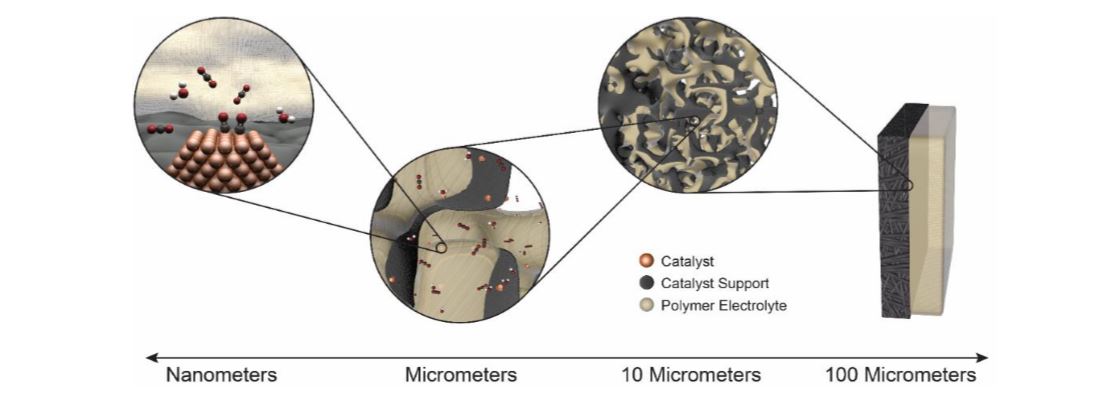
Abstract
As electrochemical technologies become increasingly important in our energy paradigm, there is a need to examine them holistically. Furthermore, for such technologies to become practical, they need to operate at high current densities to minimize various cell costs. This operating space necessitates the need for efficient transport of reactants and removal of products from the reaction site. For example, one of the main challenges towards achieving high-efficiency fuel generators performing CO2 reduction are the mass-transport limitations in traditional aqueous systems due to CO2 interactions/solubility and boundary-layer thickness. To circumvent these issues, gas-diffusion electrodes (GDEs) are considered wherein the reactants and products are fed or removed in a vapor form into a porous 3-D electrode. In this talk, we will explore the various tradeoffs endemic in GDE architectures for various electrochemical reactions including CO2 and CO reduction and O2 and H2 consumption and evolution. Such tradeoffs are quantified through multiphysics modeling of the cells including breakdowns of the various limiting phenomena at both the micro and macroscales, where the local conditions and environment around the reaction center impact reactivity and influence material development. It will be shown how transport phenomena dominate a lot of the performance and selectivity of the catalysts, which is critical to understand to improve overall performance. Different GDE design architectures will be explored and their intrinsic tradeoffs noted including complex interplay of hydration, reactant concentration, and both homogeneous and heterogeneous reactions. In particular, full vapor-phase motifs will be discussed including nonintuitive results when one goes from liquid to solid-state, polymer electrolytes. In addition, experimental data on both GDEs and model, microelectrode solid-state systems for the various electrochemical reactions will be presented and results rationalized through understanding the various concentration and ionic overpotentials.
Keywords
CO2 reduction, gas-diffusion electrode, transport phenomena, modelling, fuel cell.
Acknowledgement
This material is based upon work performed by the Joint Center for Artificial Photosynthesis, a DOE Energy Innovation Hub, supported through the Office of Science of the U.S. Department of Energy under Award Number DE-SC0004993 as well as by the Hydrogen and Fuel Cell Technologies Office of DOE through the Million Mile Fuel Cell Truck Consortium under DE-AC02- 05CH11231.
Biography
Adam Z. Weber holds B.S. and M.S. degrees from Tufts University, and a Ph.D. at University of California, Berkeley in chemical engineering under the guidance of John Newman. Dr. Weber is a Senior Scientist and Leader of the Energy-Conversion at Lawrence Berkeley National Laboratory, co-Director of the Million Mile Fuel Cell Truck Consortium and Deputy Director of HydroGen consortium. His current research involves understanding and optimizing fuel-cell and electrolyzer performance and lifetime including component and ionomer structure/function studies using advanced modeling and diagnostics, understanding flow batteries for grid-scale energy storage, and analysis of solar-fuel generators and CO2 reduction. Dr. Weber has coauthored over 150 peer-reviewed articles and 10 book chapters on fuel cells, flow batteries, and related electrochemical devices, developed many widely used models for fuel cells and their components, and has been invited to present his work at various international and national meetings. He is the recipient of a number of awards including a Fulbright scholarship to Australia, a 2012 Presidential Early Career Award for Scientists and Engineers (PECASE), the 2014 Charles W. Tobias Young Investigator Award of the Electrochemical Society, the 2016 Sir William Grove Award from the International Association for Hydrogen Energy, and a 2020R&D100 award for microlectrode development. He is a Fellow of The Electrochemical Society
Video Proceedings of Advanced Materials

Upcoming Congress



