Video Article Open Access
A Comparative Electrochemical Study of the Anticorrosion Activity of Some Sicilian Plants Extracts
Viviana Figà1,*, Maurizio Bruno2
1Euro-Mediterranean Institute of Science and Technology, Via Michele Miraglia 20, Palermo, 90100, Italy
2Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche, Università di Palermo, viale delle Scienze, Parco d’Orleans II, Palermo, 90128, Italy
Vid. Proc. Adv. Mater., Volume 2, Article ID 2021-0171 (2021)
DOI: 10.5185/vpoam.2021.0171
Publication Date (Web): 27 Jan 2021
Copyright © IAAM
Graphical Abstract
Abstract
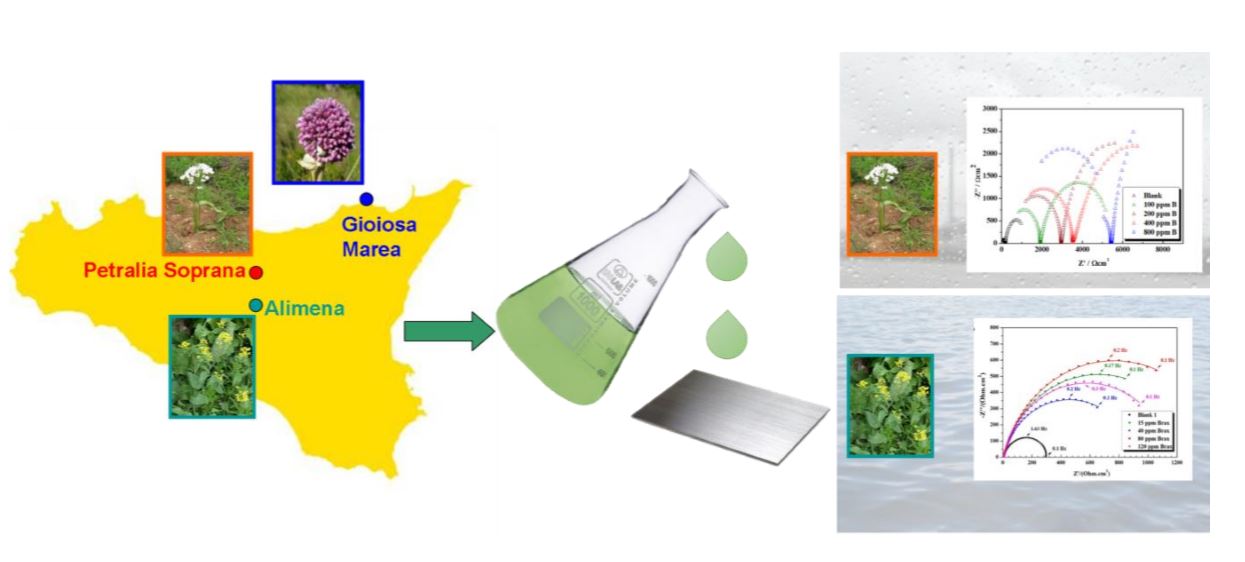
The aim of our work is to exploit the antioxidant properties of some plants for the protection of metals and alloys from corrosion, once exposed to polluted urban/industrial environments and marine areas. For this reason, we test some ethanolic extracts of plants collected in Sicily (Italy) as corrosion inhibitors of Cor-Ten steel in acidic media mimicking acidic rains and in NaCl solution mimicking seawater. In particular, we test and compare extracts of Allium commutatum, Allium subhirsutum and Brassica campestris, which grow spontaneously in our territory. The bulbs of Allium commutatum and the bulbs and aerial parts of Allium subhirsutum were collected from plants at the full flowering stage. Allium commutatum parts were reaped in Gioiosa Marea and Allium subhirsutum parts were reaped near Petralia Soprana. Brassica campestris was collected in Alimena.
We focalize on Cor-Ten steel with the aim of giving a contribution for overcoming some restrictions in its application. In fact, Cor-Ten steel is a weathering steel generally used as unpainted. It has better mechanical properties compared to other steels and a very good resistance to corrosion due to the formation of a compact oxide layer on its surface. Unfortunately, in some environmental conditions such as in polluted and marine areas, the formation of the protective layer is obstructed so that Cor-Ten cannot be employed despite its noticeable mechanical properties. In these conditions, anticorrosion coatings or corrosion inhibitors are necessary.
Plant extracts are chemically characterized by chromatography and the efficiency as corrosion inhibitors is evaluated by means of electrochemical impedance spectroscopy and dynamic polarization. In particular, different volumes of extracts are added in the corrosive solutions and the optimum concentration is evaluated for each species.
The comparison between the anticorrosion activity of bulbs extracts of Allium subhirsutum and Allium commutatum toward Cor-Ten in the acidic medium evidences slight differences. The optimum concentration is 800 ppm in both the cases; at this value, charge transfer resistances of 10.50 kWcm2 and 9.70 kWcm2 are calculated for Allium subhirsutum and Allium commutatum, respectively, corresponding to average inhibition efficiency (IE) values > 90% and < 88%.
By comparing extracts from different parts of Allium subhirsutum in acidic medium, it is found that aerial parts extracts display a lower IE with respect to bulbs extracts at the same optimum concentration. A substantial difference is noticed comparing aerial parts extracts of Allium subhirsutum and Brassica campestris. The latter has an optimum concentration at 120 ppm at which a charge transfer resistance of 1.66 kWcm2 is calculated, corresponding to a low IE of 31%. At higher concentrations, Brassica campestris extracts seem to catalyse the corrosion process in acid. Instead, they show an IE of 82% in NaCl already at 80 ppm.
In conclusion, alliaceae have proven themselves to be the best inhibitors of Cor-Ten steel corrosion in acidic environment compared to Brassica campestris extracts. Among alliaceae, bulbs extracts of Allium subhirsutum revealed the best performance. On the other hand, Brassica campestris extracts are more effective in inhibiting Cor-Ten corrosion in NaCl than in acidic solutions [1].
Keywords
Corrosion inhibitors, Cor-Ten steel, electrochemical impedance spectroscopy.
Acknowledgement
The authors would like to thank Dr. Maria Pia Casaletto for surface analysis (here not reported) as support to electrochemical data.
References
- M.P. Casaletto, V. Figà, M. Bruno, A. Napolitano, S. Piacente, Corrosion Science, 2018, 136, 91.
Biography
Viviana Figà graduated in Chemical Engineering with specialization in electrochemistry from the University of Palermo in 2004, delving into subjects such as applied electrochemistry, industrial electrochemical processes and corrosion and protection of metals. She received the Ph.D. degree in Chemical and Materials Engineering from the University of Palermo in 2009, refining basic and advanced electrochemical methods for materials science. She also graduated in Naval Engineering from the University of Napoli “Federico II” in 2017, discussing a dissertation about electrochemical devices as power generators onboard. She was a Professor of Applied Physical Chemistry at the Faculty of Science, University of Palermo, from 2009 to 2011. Her research interests regard the electrochemical deposition and characterization of inorganic and organic semiconductors to apply in optoelectronic and electrochemical devices and the study of corrosion processes specially occurring in marine environment by means of Electrochemical Impedance Spectroscopy. She is among the Editorial Board of important journals, reviewer and author of almost 30 publications about materials. She is among the scientific and local organizing committees of important international congresses and she also was the scientific leader of the project “EnzymEleSs” financed by National Research Council (CNR). Currently, she works as researcher and professional at the Euro-Mediterranean Institute of Science and Technology (IEMEST) in Palermo, Italy.
Video Proceedings of Advanced Materials

Upcoming Congress



